Saturday, May 30, 2009
Thursday, May 28, 2009
Aquarama 2009 is here! Do not miss it!
Anyway, words aside, let the pictures do the talking =)




Monday, May 11, 2009
Bukit Merah Golden Crossbacks
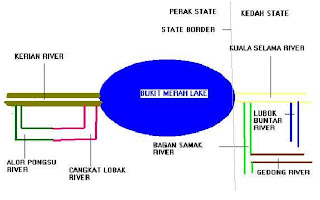
The below is a close up view of the area, detailing the different tribuaries leading to the lake as forwarded to me by Max.
The Base Colour:
Almost all Bukit Merah crossbacks are blue in base colour. The base colours range from dark blue,blue,light blue and almost silver colour. They will retain the base colour until the size of 10”-12” by then the gold frame might cover the base colour depending on the frame thickness.Some will be totally covered by the gold colouration and become Gold Base x-backs. There are also crossbacks of other base colours eg purple and green but these are few and rare
Alor Pongsu CrossBack.
The frame thickness can range from thin to thick. They will have a high chance of retaining the permanent blue base.The head are slight spoon with medium length body shape and fairly large finages.
Cangkat Lobak River CrossBack.
The frame thickness can range from thin to thick..The head are slightly spoon with medium length body shape and very small finages.The x-back in this region can grow to medium size
Gedong River CrossBack.
The frame thickness are thin.All will retain blue base when fully grown.The head are flat and long with long body shape.Finages are medium size.
Kerian River CrossBack.
The frame thickness can range from thin,thick to no frame (gold base).The head are rounded (bullet head) with long body shape and small finages.
Bukit Merah Lake CrossBack.
The frame thickness are very thick.Most will become gold base when fully grown.The head are rounded and short with short body shape and very small finages.The x-back in this region are small size ( up to 28” in length).
Chloramines and its effects on aquarium fish
Chlorine is commonly used to disinfect public drinking water. When chlorine Cl is added to water H2O, an acid known as hypochlorous acid (HOCl) is formed, it will react with ammonia present in the water to form a very stable compound known as chloramines:
HOCl + NH3 =>NH2Cl + H2O
In some cities in USA and Canada, chloramines are used as an alternative disinfectant to prevent the spread of waterborne diseases and to improve the water quality. While chloramines are not harmful to humans, chloramines have caused great concern among aquarists since chloramines are toxic to both marine and freshwater fishes. Unlike chlorine which can be easily removed, chloramines are more complex in its chemical bonding and chemically very stable.
However, when chloramines break down either naturally or through the use of dechlorination chemicals e.g. antichlorine solution, ammonia is released. This is again a toxic substance harmful to aquarium fishes. While a cycled tank with its nitrosomonas and nitrobacter bacteria colonies are able to convert harmful ammonia into less harmful nitrates, the sudden rise in the concentration of ammonia in the water might prove too much for the bacteria colonies to handle.
The harmful effect of ammonia is amplified when the pH of the water is high. This effect can be kept in check by the use of pH control tools and a bag of zeolite rocks that can remove ammonia from water. The use of zeolite to remove ammonia should used only in times of emergency e.g. ammonia spike since ammonia is a need for cultivation of bacteria for nitrogen cycle.
Coping with chloramines:
1. use aged water to remove chlorine to prevent the formation of chloramines. To prepare aged water, fill up a container with water and ensure sufficient aeration is supplied to the water to create surface disturbances. The exchange of gases caused by the disturbances will allow chlorine to diffuse out, lessening the occurrences of chloramines. It is recommended that aeration is supplied to the water for 24hrs or more for the complete removal of chlorine.
2. good maintenance of biological filter to ensure minimum level of ammonia. This can be achieved by using good quality filter media or keeping flow rate at an optimum level for bacteria to cultivate.
3. use of zeolite rocks to reduce ammonia concentration in water.
4. use of high quality activated carbon filter to remove chloramines.
References:
1. http://www.valleywater.org/Water/Water_Quality/How_we_clean_your_water/Chloramine_level/Frequently_Asked%20Questions.shtm
2. http://www.acwd.org/waterquality-chloramines.html
3. http://www.httg.com/chloramines.htm
Basic water chemistry: nitrogen cycle, GH, KH and PH
The atmosphere contains about 79% nitrogen gas (N2) and is thus a major reservoir for this important nutrient. Nitrogen is a critical component of proteins, many vitamins and the nucleic acids DNA and RNA. Plants incorporate the nitrogen from ammonia and nitrate into amino acids, proteins and nucleic acids and vitamins. These nitrogen-containing molecules are eventually consumed and are passed through the food web.
When an aquarium is first set up, the water is clean and there are no bacteria to start the nitrogen cycle. When fish and other aquatic animals are introduced to the water, the unfinished foods and their waste will decay and produce ammonia (NH3) that is highly toxic to most fishes or ammonium (NH4) which is non-toxic. These nitrogenous compounds are converted to less toxic compounds via the nitrogen cycle. When the ammonia in your aquarium is of a certain concentration, nitrosomonas bacteria will form colonies in your filter to oxidise NH3 and NH4 to nitrites (NO2) which is toxic. Nitrobacter bacteria will then oxidise the nitrites to non-toxic nitrates (NO3). Denitrification will then reduce nitrates (NO3) nitrogen (N2) or nitrous oxide (N2O) gas that will then diffused back into the atmosphere to close the nitrogen cycle. Therefore, cycling the tank refers to the process of establishing bacterial colonies in the filter bed that convert ammonia to nitrite to nitrate.
During the cycling process, ammonia levels are very high until the nitrosomonas bacteria are established to convert the ammonia into nitrites. When nitrosomonas are first established, the nitrite levels will increase until nitrobacters are established to convert the nitrites into nitrates. Thus, the water is highly toxic before the formation of nitrobacters as the ammonia and nitrite levels are very high and it is advisable to introduce fishes such as guppies or feeder goldfishes to start the nitrogen cycle in your tank before placing the arowana into the tank. Once the nitrogen cycle is set up in your tank, it is a continuous process.

General hardness (GH)
General hardness (gH) refers to the concentration of magnesium and calcium ions in water. Some species of fishes require soft water whereas others prefer hard water. Although most fishes can adapt to waters of different gH, their ability to breed may be affected. gH is generally measured either in degrees hardness or in ppm (parts per million)
| ppm | degrees | definition |
| 0 – 50 | 0 – 3 | soft |
| 50 - 100 | 3 – 6 | moderate soft |
| 100 - 200 | 6 – 12 | slight hard |
| 200 – 300 | 12 – 18 | moderate hard |
| 300 – 450 | 18 – 25 | hard |
| 450+ | 25+ | extreme hard |
Carbonate hardness (kH)
Carbonate hardness (kH) refers to carbonate hardness is directly related to the concentration of bicarbonates or carbonate ions in the water. It measures the buffering ability of the water to absorb and neutralize the addition of acid while keeping pH stable. Water with high kH has sufficient buffering to keep the pH constant since nitrogen cycle in the tank produces nitrate that forms nitric acid. Without buffering, pH will fluctuate. However, if the water has high pH and kH, it will be more difficult to lower the pH since the water is now more resistant to changes in pH. Lowering the kH of water can be done by using reverse osmosis water or by injecting carbon dioxide in the water. Increasing kH will often require the addition of sodium bicarbonate. Soft water results in low pH since there are less bonding of bicarbonate ions and hydrogen ions and hard water results in high pH.
Power of hydrogen (pH)
pH is also known as the power of hydrogen and it measures the concentration of hydrogen ions in the water. Although water is generally considered to be a stable compound, individual water molecule can gain or lose hydrogen atoms:

In pure water, there is an equal concentration of hydrogen ions (H+) and hydroxide ions (OH-) and has a pH of 7. Dissolved chemicals and minerals change the balance of those ions from a perfectly neutral state. Increase the amount of hydrogen ions and the water becomes more acid and hence a lower pH. Increase the amount of hydroxide ions will make the water becomes more alkaline with a higher pH e.g. a sudden increase in the pH often indicates high ammonia content in the water.

PH scale as seen using universal indicator
The kH of the water must also be considered because ph in harder water are more difficult to adjust. Some ways to reduce pH includes putting in driftwood into the aquarium or injecting carbon dioxide whereas putting limestones based rocks will increase the pH. However, it must be noted that pH should be gradually changed because sudden changes in pH can cause extreme stress to the fish and it might die.
Conclusions
Understanding the chemistry of the water in the aquarium is important in maintaining the well being of your fish. While nitrogen cycle, gH, kH and pH are quite distinct, they are actually interact to influence the water conditions. Decorations and the setups such as driftwood, driftwood, limestone will also affect the water parameters.